-
Hitec Medical FDA training -FDA’s Definition of Medical Devices – Part 2
FDA’s control over different categories of medical devices Label requirements “Registering a factory for a device or obtaining a registration number does not necessarily mean formal approval of the factory or its products. Any description that creates the impression that registration...Read more -
Hitec Medical FDA training -FDA’s Definition of Medical Devices
Hitec Medical FDA training -FDA’s Definition of Medical Devices FDA’s Definition of Medical Devices Medical devices refer to instruments, devices, tools, machinery, instruments, insertion tubes, in vitro reagents, or other related items that meet the following conditions, including co...Read more -
Hitec Medical FDA training – Introduction to FDA regulations
Hitec Medical FDA training – Introduction to FDA regulations Code of Federal Regulations (CFR) CFR is the integration of general and permanent rules published and published by federal government agencies and departments in the Federal Register, with universal applicability and legal effect...Read more -
ARAB HEALTH
From January 29 to February 1, 2024, the 49th Arab Health 2024 International Medical Equipment Exhibition was officially held at the Dubai World Trade Center. It is the largest international professional medical equipment exhibition in the Middle East, with a complete range of exhibits and good e...Read more -
2024 Arab Health
Welcome to visit Hitec Medical at Hall 8 G38, 2024 Dubai Arab Health. Hitec is a professional manufacturer of Respiratory, Anesthesia, Urological and Infusion therapy products. Hitec is ISO13485 registered, US FDA listed, and to be MDR CE certified soon. Sincerely hope Hitec Medical will be one ...Read more -
Hitec Medical MDR training – Technical documentation requirements under MDR(Part 2)
Hitec Medical MDR training – Technical documentation requirements under MDR(Part 2) Clinical evaluation requirements under MDR Clinical evaluation: Clinical evaluation is the collection, evaluation, and analysis of clinical data through a continuous and proactive approach, utilizing suf...Read more -
Hitec Medical MDR training – Technical Documentation Requirements under MDR (Part 2)
Hitec Medical MDR training – Technical Documentation Requirements under MDR (Part 2) Clinical evaluation requirements under MDR Clinical evaluation: Clinical evaluation is the collection, evaluation, and analysis of clinical data through a continuous and proactive approach, utilizin...Read more -
Hitec Medical MDR training – Technical Documentation Requirements under MDR (Part 1)
Hitec Medical MDR training – Technical Documentation Requirements under MDR (Part 1) Elements Content Device description, included software and accessories General description of the product, including intended use and intended users; UDI; indications and contraindications; instruction...Read more -
Hitec Medical MDR training – Product classification under MDR (Part 2)
Hitec Medical MDR training – Product classification under MDR (Part 2) Rule 10. Diagnostic and testing equipment Equipment used for lighting (examination lamps, surgical microscopes) Class I; For imaging of radiopharmaceuticals in the body (gamma camera) or for direct diagnosis or detec...Read more -
Hitec Medical MDR training – Product classification under MDR (Part 1)
Product classification under MDR Based on the intended use of the product, it is divided into four risk levels: I, IIa, IIb, III (Class I can be subdivided into Is, Im, Ir, according to actual conditions; these three categories also require third-party certification before obtaining a CE certific...Read more -
Hitec Medical MDR training -Definition of MDR Terms (Part 2)
Hitec Medical MDR training -Definition of MDR Terms (Part 2) Intend use The manufacturer designates the use in clinical evaluation based on the data provided in labels, instructions, promotional or sales materials, or statements. Label Printed text or graphic information that appears o...Read more -
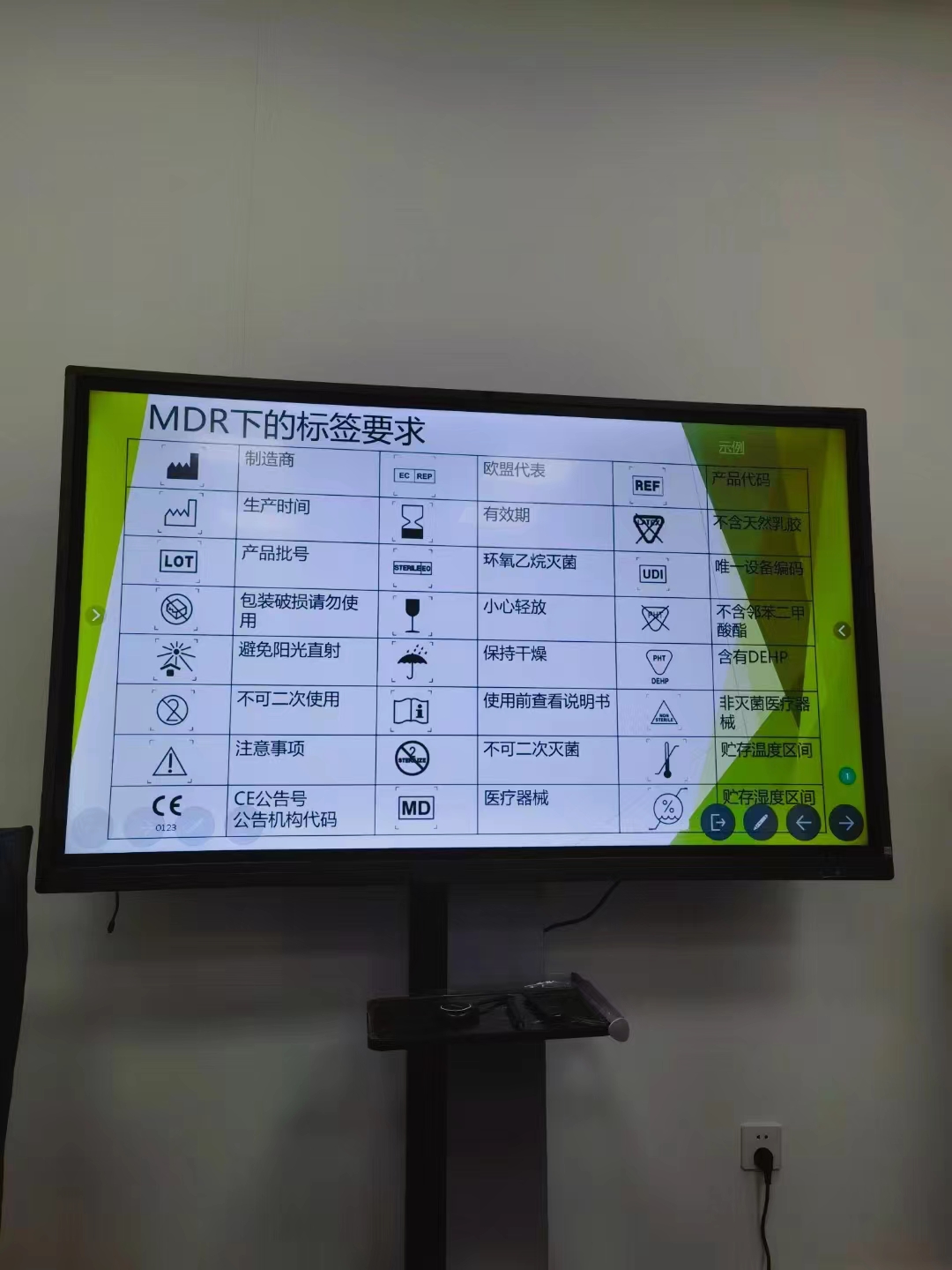
Hitec Medical MDR training -Definition of MDR Terms
Hitec Medical MDR training – Definition of MDR Terms Medical device It refers to any instrument, equipment, appliance, software, implant, reagent, material, or other item used solely or in combination by a manufacturer for one or more specific medical purposes in the human body: Diagnosis,...Read more





