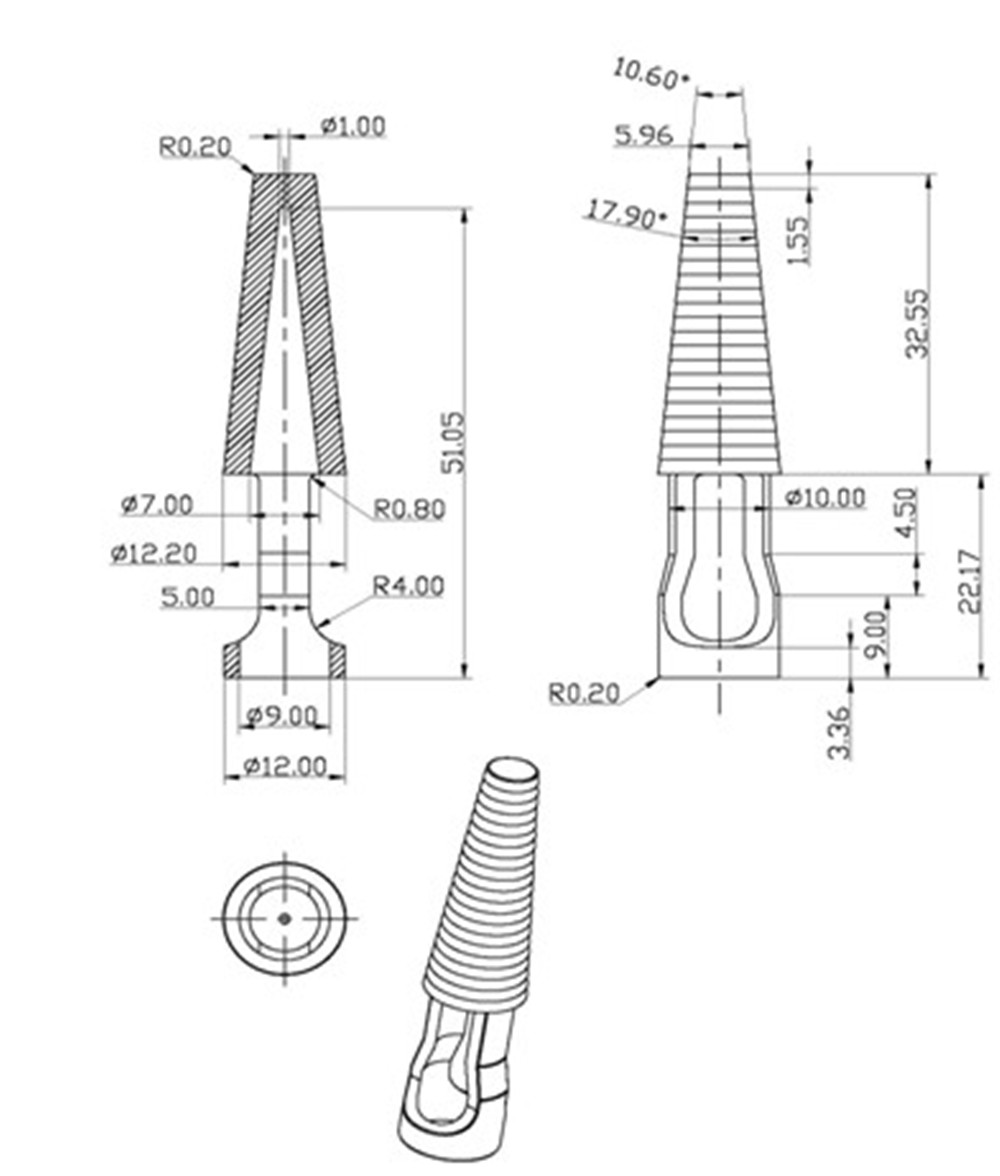
- Universal size is molded to fit all sizes of catheters or tubes.
- Graduated design made it to easily accommodate tubing of various internal diameters.
- Ribbed designed provides strong, secure connections.
- Ergonomic flanged designed assists withdrawal and maximize grip
- Can be supplied sterile in individual peel pouches, or even in bulk packaging.
- Made from non-toxic PP
- EO Sterile, single use only
- 100% latex-free